Multifarious Effect of Butyrate on Intestinal Function
Feedinfo, November 2013
Butyrate deserves particular attention as an important energy source for intestinal epithelial cells and its multiple beneficial effects on vital intestinal function. In the digestive tract, butyrate is naturally present in high concentrations in the lumen of the large intestine.
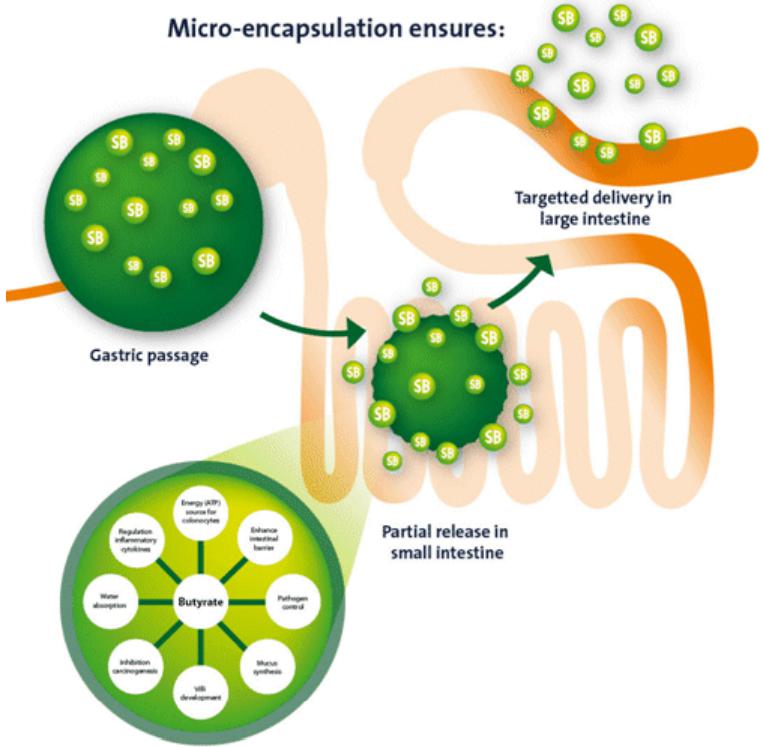
Dietary fibers are used in diets for companion animals as a substrate for the microbial production of butyrate. Microbial fermentation of dietary fiber in the colon results in the production of shortchain fatty acids (SCFA’s), such as acetate, propionate and butyrate. The prebiotic potential of different fiber sources is frequently compared based on microbial production of SCFA’s and in particular butyrate. The positive effect of butyrate can also be achieved by direct addition of the SCFA to the diet. However, unprotected butyrate will be directly absorbed in the first part of the digestive tract before reaching the large intestine. Micro-encapsulation of butyrate results in the targeted release of butyrate over the whole digestive tract and, just as importantly, correct coating reduces the typical unpleasant smell of butyrate.
SCFA’s
The intestinal microbiota plays a critical role in the establishment and maintenance of intestinal health. Fermentation of dietary fibers by commensal bacteria results in the production of SCFA’s. Approximately 95-99% of SCFA’s produced in the hindgut is quickly absorbed and delivers energy to the animal (Von Engelhardt, et al. 1989). Dietary fiber and their fermentation metabolites play an important role in the metabolism and health of companion animals, even in cats with their carnivorous nature (Rochus, et al. 2013).
Butyrate is considered the most important SCFA for intestinal epithelial cells. Especially the cells in the colon take up butyrate where it is used for the production of energy and the maintenance of colonic homeostasis (Von Engelhardt, et al. 1998). Numerous studies indicate the importance of butyrate for the provision of energy to epithelial cells, sodium and water absorption, influence on epithelial cell proliferation and differentiation, villi development and in improving gut defense systems. Butyrate strength the barrier function, have antimicrobial potency and positively influence immune system. Reviewed by Guilleteau et al. (2010) and Hamer et al. (2008).
The lumen of the colon has the highest density of microbes in the body and specific bacteria belonging to genera such as Clostridium, Eubacterium and Butyrivibrio ferment dietary fiber into butyrate at very high concentrations (Donohoe, et al. 2011). The quantity of butyrate produced in the colonic lumen depends on the composition of the microflora and the amount of fermentable substrates available in the diet. Higher levels of butyrate can be achieved by adding fermentable fibers to the diet. However diets with too large amounts of fermentable fibers can have negative effects on fecal characteristics such as loose stools and flatulence (Gross et al. 2000). Intestinal infections, chronic inflammatory disorders and use of antibiotics negatively affect the colonic microflora and may result in reduced production of butyrate (Kumari, Ahuja en Paul 2013). The addition of microencapsulated butyrate to the diet is a solution to provide additional amounts of butyrate to the colon (Guilloteau, et al. 2010).
Energy source for intestinal cells
Energy supply to intestinal epithelial cells is important due to the fact that the mammalian intestinal mucosa is one of the most rapidly replicating tissues in the body. For example, studies in rodents demonstrate that the small intestinal epithelium is completely replaced every two to three days (Ziegler, et al. 2003). Thereby, the colonic epithelium is permanently in close association with microbes and their product. There is a constant immunological challenge, which makes the barrier defense function of the epithelial layer of great importance (Hamer, et al. 2008). The dynamic processes of intestinal cells (proliferation, migration, differentiation and apoptosis) and defense function are markedly influenced by the type of diet and the adequacy of specific nutrients in the diet (Ziegler, et al. 2003) (Peng, et al. 2009).
In the small intestine microbial formation of butyrate is low or absent. Nevertheless, cells in the small intestine also express the transporter to bring butyrate into the cells. Claus et al. (2006) fed butyrate to healthy growing piglets and demonstrated that targeting butyrate into the small intestine improves villi development, gut morphology and function.
Further on in the digestive tract, butyrate represents the preferred energy-providing substrate for the colonic cells. Whereas most other cell types utilize glucose as their primary energy source, colonocytes have a great capacity to rapidly metabolize butyrate. After transport into the colonic cells, β-oxidation in the mitochondria results in acetyl-CoA, which goes into the citric acid cycle and proceeds in the production of adenosine triphosphate (ATP) with CO2 as a byproduct (Donohoe, et al. 2011). Otherwise butyrate is, after acetate, a major precursor for de novo lipid synthesis. High proportion of these synthesized lipids is used for incorporation into the cell membrane (Zambell, Fitch en Fleming 2003). As such, the synthesis of many key components of the intestinal epithelial tissue depends on butyrate metabolism. Only small quantities of butyrate will reach the general peripheral blood circulation and liver (Guilloteau, et al. 2010).
At an early stage of life butyrate is an important factor in the development of the gastrointestinal tract. Newborn animals produce small quantities of butyrate, the production increases during the development of the hindgut (Guilloteau, et al. 2010).
Supplementation of milk formula and starter diets with butyrate contribute to enhanced development of the intestinal mucosa and improvement of digestive processes in neonatal calfs and piglets (Gorka, et al. 2009) (Kotunia, et al. 2004)
Cell growth and proliferation; the ‘butyrate paradox’
Cells in the large intestine particularly use butyrate as energy source for cell metabolism and it stimulates colonocyte proliferation and differentiation both in vivo and in vitro (Ziegler, et al. 2003). In contrast to the trophic effect on normal healthy colonocytes, butyrate decreases proliferation and differentiation of tumor cell lines and can finally induce apoptosis; programmed cell death (Guilloteau, et al. 2010) (Hesta, Arnouts en Janssens 2008) (Ziegler, et al. 2003). Butyrate has the ability to influence the gene expression, thereby inducing early apoptosis in cancer cells. Its opposite is seen in normal cells, where butyrate activates gene expression for cell proliferation and maturation (Canani, Di Costanzo, et al. 2011)
Several epidemiological studies support the protective role of dietary fiber and its breakdown product butyrate against colonic cancer. These contradictory patterns of butyrate of stimulating cell growth and differentiation of normal cells and apoptosis (early cell death) of tumor cells, represents the so called ‘butyrate paradox’ (Canani, Di Costanzo, et al. 2011).
Colonic water absorption and antidiarrheal agent
The absorption of SCFA’s has a significant impact on the absorption of sodium, potassium, water and on the electrolyte balance in general. Transport mechanism of SCFA’s involves Na+ and H+ uptake in cells. Herschel et al (1981) determined that SCFA’s and Na+ were rapidly absorbed, and at the same rate, in the colonic mucosa of dogs. This results in osmotic changes and increased absorption of water from the colon to the epithelial cells (Von Engelhardt, et al. 1998) (Canani, Di Costanzo, et al. 2011).
Clausen et al (1991) found a correlation between antibiotic-associated diarrhea and reduced fecal concentrations of butyrate from microbial fermentation. The researchers suggest that the diarrhea might be a secondary effect coming from impaired colonic fermentation, resulting in the accumulation of luminal fibers and/ or reduced SCFA’s stimulated water absorption. Scarpellini et al (2007) provided fat-coated butyrate to patients with diarrhea-predominant irritated bowel syndrome. The treatment resulted in improved fecal consistency (reduction of fecal moisture) and reduced frequency of daily stools. Symptoms as abdominal pain, meteorism (bloating) and flatulence were significantly reduced after 30 days of butyrate administration.
Inflammatory status
Butyrate has a role as an anti-inflammatory agent, primarily via inhibition of nuclear factor kB (NFkB) activation in colonic epithelial cells. NF-kB regulates many cellular genes involved in early inflammatory responses (Canani, Di Costanzo, et al. 2011) (Canani, Di Costanzo en Leone 2012). The activity of NF-kB is frequently deregulated in colon cancer and in inflammatory bowel diseases (IBDs). Clinical studies show beneficial effects of butyrate administration, directly or via dietary fiber, on inflammation and symptoms in patients with ulcerative colitis (UC, form of IBD). Di Sabatino et al. (2007) demonstrated that the oral butycoat is effective in the treatment of mild to moderate Crohn’s disease. A daily dose of oral butyrate resulted in reduction of the clinical signs and inflammatory parameters in the large intestine.
Barrier function and antimicrobial activity
Extensive use of antibiotics leads to the emergence of antimicrobial resistance, which is not only an issue in livestock, but also in pet animals. Companion animals are in close contact with humans which increase the risk on zoonotic transmission, moreover, difficult to treat infections, use of antimicrobials which are also important in human medicine and limited control on antibiotic treatments increase concerns for public health. Groups of opportunistic pathogens in pet animals include staphylococci, enterococci, Escherichia coli and Salmonella (Weese 2008).
Antimicrobial peptides (like defensins and cathelicidins) are essential effector molecules of the innate immune system and are of great importance in bacterial host defense. These peptides, also mentioned host defense peptides (HDP’s), possess broad-spectrum antimicrobial activities against bacteria, protozoa, enveloped viruses and fungi. HDP’s can bind to different types of microbial membranes, cause membrane disruption which results in microbial death (Tizard 2009). This first line of defense and their natural broad-spectrum against microbes, makes the up regulation of HDP’s an interesting approach as alternative or adjunct therapy to antibiotic treatment.
A recent study of Sunkara et al (2011) revealed a novel role for butyrate in the host defense system. Results from this study in chickens revealed that butyrate increases the antibacterial activity of host immune cells by up-regulation of an array of HDP’s in vitro and in vivo. This immune defense was coupled with a minimum impact on immune cell activation and inflammation. More importantly, oral supplementation of butyrate resulted in a significant reduction of Salmonella enteritidis after infection.
Same results of butyrate were seen in other studies in mammels. After antibiotic treatment of Shigella-infected patients, adjunct therapy of sodium butyrate significantly increased the expression of a specific HDP (cathelicidin LL-37) in the rectal epithelium. Accompanied by early improvement of rectal inflammation and reduction of proinflammatory cytokines in the stool compared to the control group (Raqib, Sarker en Mily, et al. 2012). Oral butyrate treatment in Shigella infected rabbits resulted in reduced clinical illness, less severity of inflammation in the colon, significant up-regulation of antimicrobials (HDP’s) in the gut and early reduction of Shigella ¬count in the faeces (Raqib, Sarker en Bergman, et al. 2006).
Another mechanistic explanation on the antimicrobial efficacy of butyrate is discussed by the research group of Van Immerseel (2006). An early step in the pathogenesis of bacteria, like Salmonella, is the interaction between the bacteria and host cells. At low doses butyrate down regulates expression of invasion genes in Salmonella, thereby reducing the ability of the bacteria to attach to host cells of the intestinal epithelium, becoming invasive and virulent (Van Immerseel, De Buck, et al. 2004) (Gantois, et al. 2006). Other studies confirm the efficacy butyrate on inhibition of pathogen colonization of E. coli (Panda, et al. 2009) and in particular Salmonella (Van Immerseel, Fievez, et al. 2004) (Van Immerseel, Boyen, et al. 2005) (Boyen, et al. 2008) .
Finally as major precursor for de novo lipids synthesis used for the cell membrane, butyrate contributes the maintenance of barrier and transporter functions of the gut (Zambell, Fitch en Fleming 2003). Especially for butyrate there are indications that it increases the defense barrier of the colon mucosa by stimulation of the formation of mucin glycoproteins. These mucin glycoproteins are essential in the mucus layer which protects the intestinal epithelium (Leonel en Alvarez-Leite 2012) (Havenaar 2011). Moreover Ma et al. (2012) observed an important role of butyrate in intestinal wound healing through its positive effect on the tight junctions and gut integrity.
Coating
Butyric acid is available as the Na, K, Mg or Ca salt (Guilloteau, et al. 2010). Uncoated butyrate is directly available and will immediately be absorbed in the first part of the digestive tract before reaching the large intestine. In order to exert the influence in the large intestine, dietary butyrate should slowly be released over the gastro intestinal tract (Hu en Guo 2007).
The aim of a coating or encapsulation is to have a targeted release in the whole digestive tract. The protective lipid matrix used for microencapsulation of organic acids allows slow-release of active ingredients over the digestive tract, preventing the immediate disappearance of the ingredient (Piva, et al. 2007).
In swines, as well as in chickens, it is shown that coated butyrate is superior to uncoated butyric acid in reducing intestinal Salmonella colonization (Van Immerseel, Boyen, et al. 2005) (Boyen, et al. 2008). Thereby environmental contamination and transmission of Salmonella to other uninfected animals was reduced in the coated butyrate group. The researchers suggest that uncoated butyrate is taken up by the cells in the upper digestive tract, where as coated butyric acid will influence the colonization of Salmonella bacteria at the site of the colonization, i.e., in the gut (Van Immerseel, Boyen, et al. 2005) (Van Immerseel, Fievez, et al. 2004).
The type of coating influences the release of butyrate over the digestive tract. Proper coating should not be decomposed in the stomach but gradually released in the presence of fat degrading enzymes. By the use of a dissolution test the release rate of sodium butyrate is tested and analyzed. This is done by simulating the same conditions of the stomach and intestinal tract. By testing Excential Butycoat, all butyrate is passing the stomach and is gradually released in the intestinal fluid (Internal research, 2012).
In addition to targeted release of SCFA over the digestive tract, coating reduces the typical unpleasant smell of butyrate. Although preferred by some animal species, most pet owners do not prefer the smell of butyrate in pet food. Wageningen University tested the effect of extrusion on odor properties of Excential Butycoat (micro-encapsulated sodium butyrate 30%) in a commercial dog food formulation. During the extrusion process and after packaging of the kibbles, there was no clear difference observed in smell between the control diets and diets with the coated butyrate (Poel, van der 2013).
Conclusion
Butyrate is essential as an energy source for epithelial cells and vital intestinal function. Diverse beneficial effects of butyrate on animal health can be achieved by the use of dietary fibers and by direct addition of butyrate to the diet. Use of proper micro-encapsulated butyrate, like Excential Butycoat, results in targeted release of the SCFA over the digestive tract, moreover, a reduction of the typical smell of butyrate during production and in the feeding of butyrate containing diets to pet animals.
References on request
